How well did it work?
The Ru/CeO2 catalysts with 2 wt% Ru was prepared using a wet impregnation method. Three different CeO2 supports with the rod, particle, and cube morphologies were produced. Then the catalysts were thoroughly studied using PXRD, ICP-OES, TEM, XPS, and TPR for the analyses of their morphological and electronic properties.
Metal Nanoparticle Sizes
TEM images and Ru size distribution of 2 wt% reduced Ru/rod-CeO2 (a, b and c), Ru/particle-CeO2 (d, e and f) and Ru/cube-CeO2 (g, h and i). All suppots show a lattice spacing of 0.31 nm assigned to the (111) lattice plane, which may be induced by the high temperature calcination (600 C).

Material Reducibility
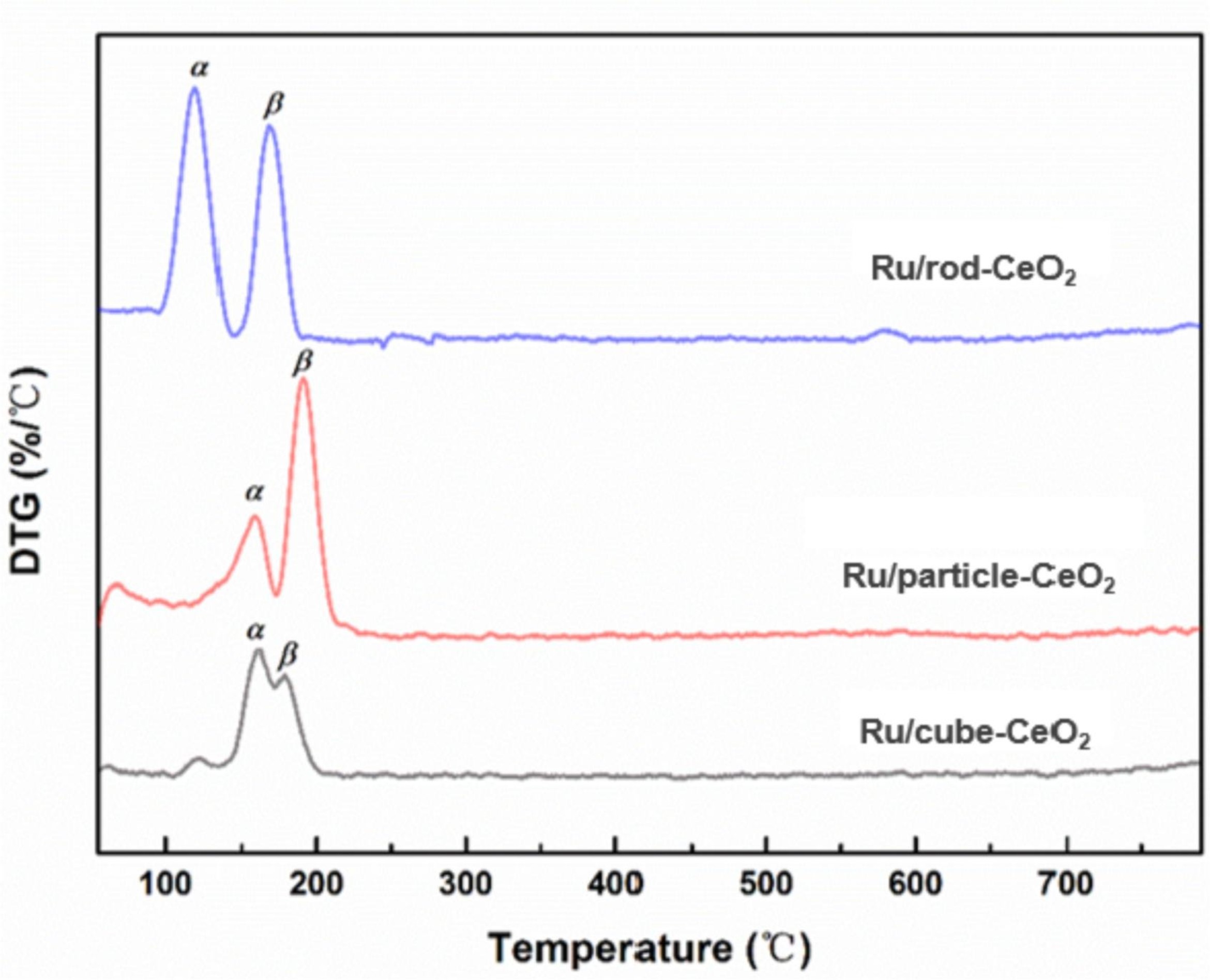
The reducibility of Ru/CeO2 materials was studied using H2 temperature programmed reduction (H2-TPR). The reduction peaks (peak α) at low temperature are related to smaller particle size accomplished with Ru-O-Ce bonds, whilst the ones (peak β) at higher temperature are associated to bigger RuOx clusters composed of Ru-O-Ru bonds. Ru/rod-CeO2 with more intense peak α suggests more abundant support-metal-interaction (Ru-O-Ce species).
Catalytic Performance
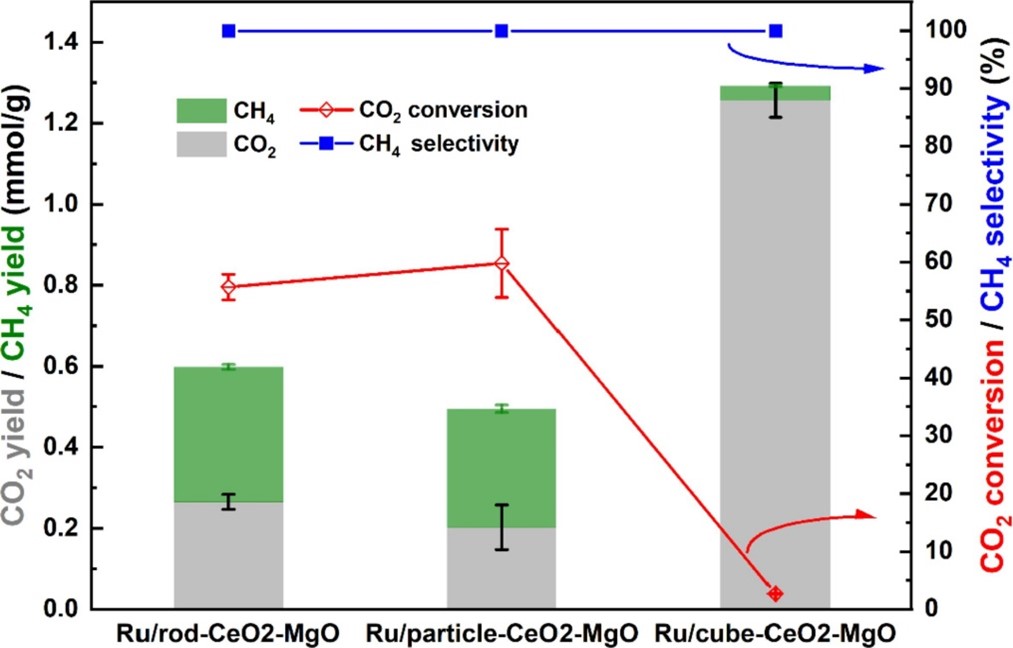
The desorption capacity of C1 species over combined materials was evaluated using the sum of CO2 and CH4 yield. The desorption capacity of C1 species over combined materials, representing the CO2 throughput, was evaluated using the sum of CO2 and CH4 yield.
How did XPS help?
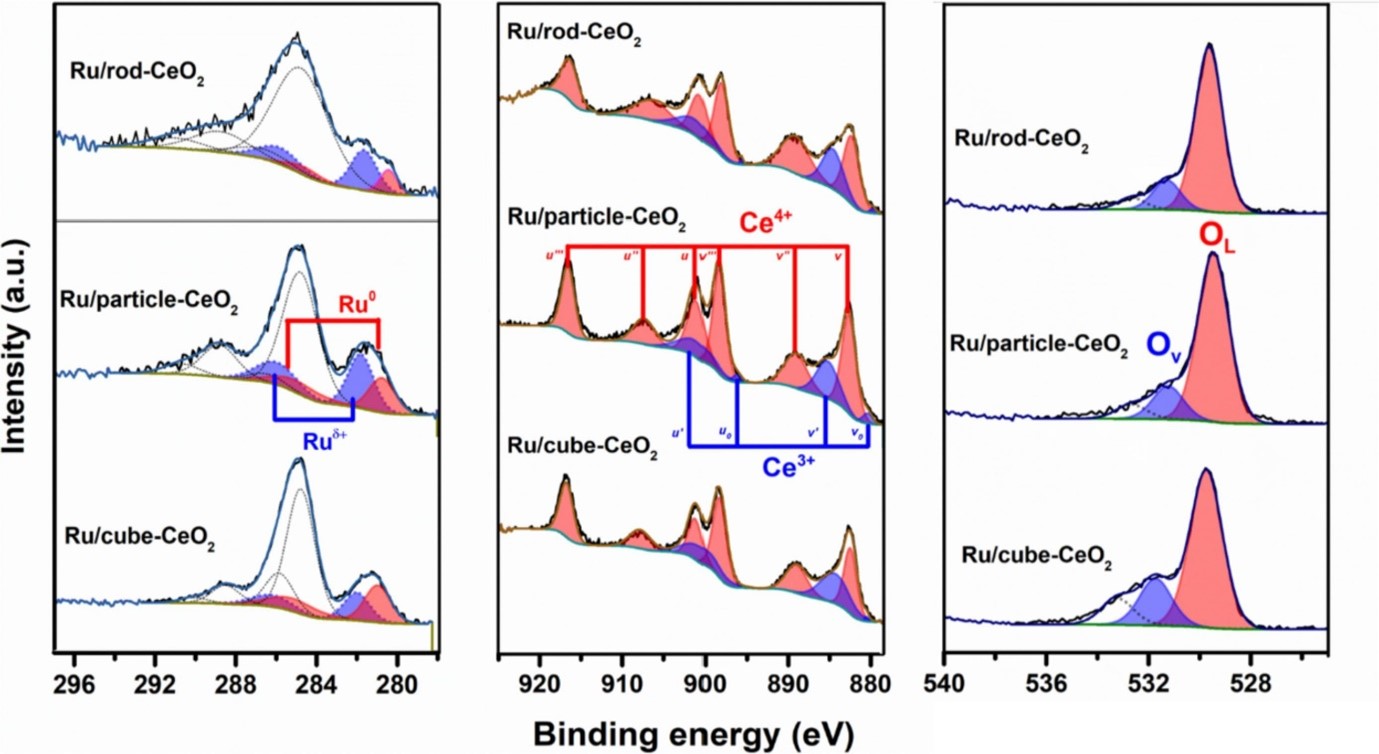
XPS results indicated that Ru/rod-CeO2 contains the highest Ru+/(Ru0 + Ru+), suggesting the most abundant support-metal interaction (SMI) and well-dispersed Ru (consistent with H2-TPR results), which is advantageous in the catalytic reaction. The spectra also show the Ru/cube-CeO2 catalyst has the highest Ce3+/Ce4+ ratio and the most abundant oxygen vacancy defects (OV/OL ratio) compared with the other two catalysts, which is promoting CO2 adsorption by forming bicarbonates, and subsequently enhancing the conversion.